Here's a news article from Nature:
The lightning-fast quest for COVID vaccines — and what it means for other diseases.
The speedy approach used to tackle SARS-CoV-2 could change the future of vaccine science. by Philip Ball
"The research that helped to develop vaccines against the new coronavirus didn’t start in January. For years, researchers had been paying attention to related coronaviruses, which cause SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome), and some had been working on new kinds of vaccine — an effort that has now paid off spectacularly.
"Conventional vaccines contain viral proteins or disabled forms of the virus itself, which stimulate the body’s immune defences against infection by a live virus. But the first two COVID-19 vaccines for which efficacy was announced in large-scale (phase III) clinical trials used just a string of mRNA inside a lipid coat. The mRNA encodes a key protein of SARS-CoV-2; once the mRNA gets inside our cells, our bodies produce this protein. That acts as the antigen — the foreign molecule that triggers an immune response. The vaccines made by Pfizer and BioNTech and by the US pharmaceutical company Moderna both use mRNA that encodes the spike protein, which docks to human cell membranes and allows the coronavirus to invade the cell.
...
"The approach has matured just at the right time; five years ago, the RNA technology would not have been ready.
...
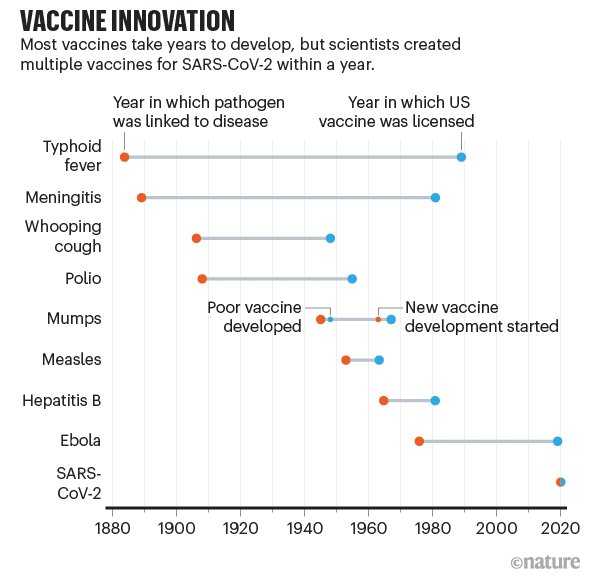
"The slowest part of vaccine development isn’t finding candidate treatments, but testing them. This often takes years (see ‘Vaccine innovation’), with companies running efficacy and safety tests on animals and then in humans. Human testing requires three phases that involve increasing numbers of people and proportionately escalating costs. The COVID-19 vaccines went through the same trials, but the billions poured into the process made it possible for companies to take financial risks by running some tests at the same time."
HT: Muthu Muthukrishnan



No comments:
Post a Comment